Most people assume that if a drug is approved by the FDA or similar agencies, it’s completely safe. But the truth is, some of the most dangerous drug interactions aren’t found until after millions of people have started taking the medicine. These are called post-market drug interactions - and they’re more common than you think.
Why Clinical Trials Miss Dangerous Interactions
Before a drug hits the market, it goes through clinical trials. These studies usually involve between 1,000 and 5,000 people, last 6 to 12 months, and focus on healthy adults or those with a single condition. That’s not the real world. Real patients take multiple medications. They’re older. They have diabetes, kidney disease, or heart failure. They drink grapefruit juice. They take herbal supplements like St. John’s Wort. None of that shows up in the trials. Take simvastatin (Zocor), a common cholesterol drug. In trials, it looked fine. But after millions of people started using it, doctors noticed something alarming: when taken with certain antifungal drugs like fluconazole, it caused severe muscle damage - even kidney failure. Why? Fluconazole blocks the liver enzyme (CYP3A4) that breaks down simvastatin. Blood levels of the statin skyrocketed - by 3 to 10 times. That’s not a small risk. It’s life-threatening. Grapefruit juice does the same thing with atorvastatin (Lipitor). One glass can raise drug levels by up to 15 times. That’s not a myth. That’s science. And it wasn’t clear on the label until after people started ending up in the ER.What Happens When a Drug Goes Public?
Once a drug is on the market, the real monitoring begins. This is called post-marketing surveillance. Systems like the FDA’s FAERS (FDA Adverse Event Reporting System) collect reports from doctors, pharmacists, and even patients. In 2020, a study found that nearly 20% of new drugs got a “black box” warning - the strongest safety alert - after they were already being sold. Four percent were pulled off shelves entirely. One of the most shocking cases was benfluorex (Mediator), a weight-loss drug sold in France for over 30 years. It was linked to heart valve damage in 5 million people. The risk only became obvious after decades of use. By the time it was withdrawn in 2009, hundreds had died. Another example is pergolide, a Parkinson’s drug. After about 1 million patient-years of use, it was tied to heart valve problems. The FDA pulled it in 2007. These aren’t rare accidents. They’re predictable failures of early testing.Types of Post-Market Interactions
Not all interactions are the same. They fall into three main categories:- Drug-drug interactions: One medication changes how another works. Like when the antibiotic clarithromycin increases levels of the blood thinner apixaban (Eliquis), raising bleeding risk.
- Drug-condition interactions: A health problem makes a drug more dangerous. For example, someone with liver disease may not clear a drug like diazepam (Valium) properly, leading to dangerous sedation.
- Drug-food interactions: What you eat or drink changes how your body handles the drug. Grapefruit juice is the classic, but pomegranate, Seville oranges, and even charcoal can interfere with medications.
These aren’t just theoretical. FAERS data from 2015 to 2020 showed 2,847 reports of rhabdomyolysis - a condition where muscle tissue breaks down - tied to statin interactions. Over a third of those involved simvastatin and antifungals. One Reddit user wrote: “My doctor didn’t warn me about grapefruit with my Lipitor. I ended up in the ER with kidney damage.”
How We Catch These Interactions Now
The system isn’t perfect, but it’s better than it used to be. The FDA’s Sentinel Initiative, launched in 2008, now tracks over 300 million patient records from hospitals, insurers, and clinics. It looks for patterns - like a sudden spike in kidney failure among people taking Drug A and Drug B together. Pharmacists use tools like the Naranjo Algorithm to judge whether a reaction is likely caused by a drug interaction. It scores factors like timing, whether symptoms improved when the drug was stopped, and whether other causes exist. It takes training - about 9 hours - to use it properly. Tech is catching up too. Oracle Health Sciences launched an AI system in January 2023 that can scan 10,000 adverse event reports daily with 92.7% accuracy. The EU’s EudraVigilance system now uses machine learning to spot signals in just 45 days - down from 18 months. The FDA also has a Drug Interaction API that handles 2.5 million queries every day from electronic health records. When a doctor prescribes a new drug, the system can flag known dangerous combos before the patient even walks out the door.Why This Still Isn’t Enough
Here’s the hard truth: we’re missing most of these events. Experts estimate that 90 to 95% of adverse reactions go unreported. Why? Patients don’t connect their muscle pain to a new pill. Doctors assume it’s just aging. Pharmacies don’t always have time to dig deep. And even when we know about an interaction, the warning labels often suck. Take apixaban and St. John’s Wort. The FDA issued a safety alert in 2022 after a 78-year-old had life-threatening bleeding after combining them. But the drug’s label still doesn’t clearly say: “Don’t take this with herbal supplements.” A 2021 Duke University study found that 15 to 20% of hospital admissions in the U.S. are due to bad drug interactions. That’s preventable. But without standardized, easy-to-read warnings, patients and providers are flying blind.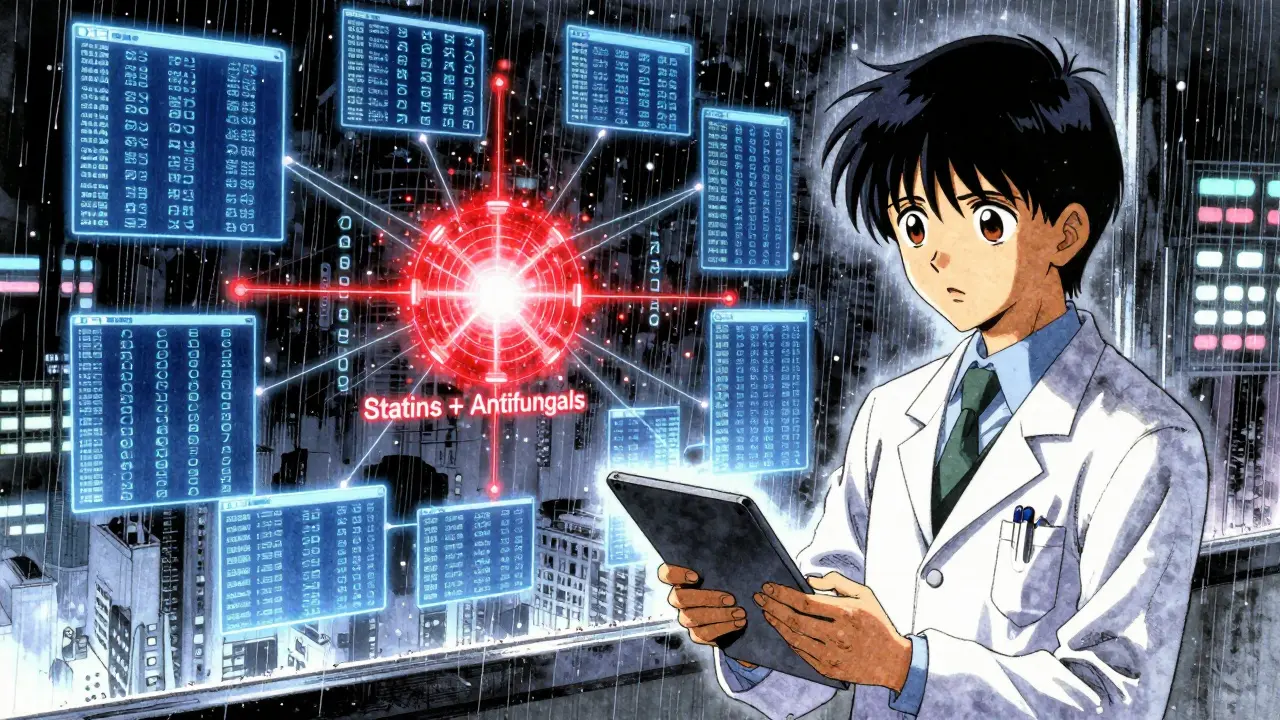
What This Means for You
If you’re on more than one medication - especially if you’re over 65, have chronic illness, or take supplements - you’re at risk. Here’s what you can do:- Always tell your doctor and pharmacist about everything you take - including vitamins, herbs, OTC painkillers, and even recreational substances.
- Use a free interaction checker like GoodRx or Medscape. One user on Trustpilot wrote: “The interaction warning stopped me from taking ciprofloxacin with my blood pressure meds. My pharmacist said it could have caused fatal heart rhythm problems.”
- Ask: “Could this interact with anything else I’m taking?” Don’t wait for them to bring it up.
- If you start a new drug and feel unusual muscle pain, dizziness, confusion, or bleeding - don’t ignore it. Call your provider. It might be the drug.
It’s not about being paranoid. It’s about being informed. The drug approval process is designed to catch the obvious dangers. But the hidden ones? Those only show up when real people use real doses over real time.
The Bigger Picture
This isn’t just a medical issue - it’s an economic one. The Institute of Medicine estimated that bad drug reactions cost the U.S. $3.5 billion a year. About a third of that - over $1 billion - comes from interactions. The global market for drug safety monitoring is now $7.3 billion and growing fast. Pharma companies are investing heavily in AI, blockchain, and real-world data because they can’t afford another scandal like thalidomide or Mediator. But the real winners? Patients who learn to ask questions. Pharmacists who double-check prescriptions. Doctors who listen. The system is designed to protect you - but it’s not perfect. You’re the last line of defense.What are post-market drug interactions?
Post-market drug interactions are harmful reactions between medications, or between drugs and food/supplements, that weren’t detected during clinical trials but appear after the drug is widely used. These include drug-drug, drug-condition, and drug-food interactions, often caused by enzyme interference like CYP3A4 inhibition.
Why weren’t these interactions found before the drug was approved?
Clinical trials are too small (usually 1,000-5,000 people), too short (6-12 months), and too controlled. They often exclude elderly patients, those with multiple health conditions, and people taking other medications. Dangerous interactions only show up when millions use the drug long-term under real-world conditions.
How common are dangerous drug interactions after approval?
About 20% of new drugs receive a black box warning after approval, and 4% are pulled from the market due to safety issues. A 2021 FDA analysis found that one-third of new drugs approved over a 10-year period had a major safety event - like a withdrawal, new warning, or public alert - after launch.
Can grapefruit juice really make my medication dangerous?
Yes. Grapefruit juice blocks the CYP3A4 enzyme in the gut and liver, which is responsible for breaking down many drugs. For example, it can raise atorvastatin (Lipitor) levels by up to 15 times, increasing the risk of severe muscle damage. Other drugs affected include some blood pressure meds, anti-anxiety drugs, and statins.
What should I do if I’m taking multiple medications?
Always give your pharmacist a complete list of everything you take - prescriptions, OTC meds, vitamins, and herbs. Use a trusted drug interaction checker like GoodRx or Medscape. Ask your doctor: “Could this interact with anything else I’m on?” Don’t assume they know. If you notice new side effects - like unexplained muscle pain, dizziness, or bleeding - contact your provider immediately.




Suresh Kumar Govindan
January 24, 2026 AT 14:47The FDA is a puppet of Big Pharma. Every approval is a calculated gamble on human lives. They don't care about you-they care about quarterly profits. The CYP3A4 enzyme? That’s not science-it’s corporate silence dressed in white coats.
They knew. They always knew.
And still, they let it happen.
George Rahn
January 26, 2026 AT 08:07Let me be perfectly clear: the American healthcare system is a grotesque cathedral of institutional incompetence. We outsource safety to algorithms while our elders choke on statins and grapefruit juice like tragic Shakespearean fools. This isn’t medicine-it’s performance art with a side of liability waivers.
And yet, we still bow to the altar of ‘FDA-approved.’ Pathetic.
Ashley Karanja
January 26, 2026 AT 10:31I’ve been a clinical pharmacist for 18 years, and I can tell you-this isn’t new, but it’s getting worse. The shift from reactive to proactive pharmacovigilance is happening, but glacially. AI tools like Oracle’s system? Game-changers. But they’re only as good as the data feeding them.
And here’s the kicker: most patients don’t even know what ‘CYP3A4’ means, let alone that grapefruit juice is a silent saboteur. We need better patient education-not just warnings buried in 12-point font.
Also, if you’re on apixaban? Please, for the love of all that’s holy, stop taking St. John’s Wort. It’s not ‘natural’ if it kills you.
And yes, I’ve seen it. I’ve seen the labs. I’ve held the hands.
We can do better. We just have to choose to.
Karen Droege
January 27, 2026 AT 16:18Oh my GOD. I just checked my meds-simvastatin and fluconazole. I’ve been taking them together for 8 months. I thought my muscle aches were from yoga.
MY PHARMACIST NEVER TOLD ME.
Are you kidding me?!
I’m calling them right now. And then I’m going to file a complaint with the state board. This is negligence. This is criminal.
Why do we still trust systems that fail us like this?
And why do we let them?
😭
Napoleon Huere
January 29, 2026 AT 13:26It’s funny how we worship science until it tells us something we don’t want to hear. We want our pills to be magic bullets-no side effects, no compromises, no consequences. But biology doesn’t care about our illusions.
Every drug is a negotiation with chaos. The body is not a machine. It’s a symphony-and one wrong note can collapse the whole thing.
Maybe the real problem isn’t the FDA. Maybe it’s our refusal to accept that nothing is truly safe. Only ‘safer.’
And ‘safer’ isn’t enough when you’re the one bleeding out in the ER.
Shweta Deshpande
January 29, 2026 AT 14:37Hey everyone, I just wanted to say-this post really hit home for me. My mom is 72 and takes 7 different meds. She also drinks grapefruit juice every morning because ‘it’s healthy.’ I didn’t even know it could be dangerous until I read this.
I’m going to sit down with her this weekend and go through every pill with her pharmacist. No more assumptions.
You’re not being paranoid-you’re being brave.
And if you’re reading this and you’re on meds? Please, just ask one question today. ‘Could this hurt me?’ It might save your life.
Love you all. 💕
Aishah Bango
January 30, 2026 AT 18:49People like you are the reason this system is broken. You blame the FDA while ignoring your own responsibility. If you don’t read the damn label, that’s on you. If you take herbal supplements without asking, that’s on you. If you think ‘natural’ means ‘safe,’ you deserve what happens to you.
Stop whining. Start reading.
And stop pretending this is a conspiracy. It’s just negligence-and you’re part of it.
Simran Kaur
February 1, 2026 AT 14:02In India, we call this ‘jugaad’-fixing things with duct tape and hope. Our elders take aspirin with turmeric, warfarin with neem, and never tell the doctor. We think tradition beats science.
But science doesn’t care about tradition. It only cares about enzymes.
I’ve lost two cousins to drug interactions. One from a common painkiller mixed with an ayurvedic tonic. No one warned them. No one knew.
This isn’t just an American problem. It’s a human problem.
We need community pharmacists in every village. We need simple, loud, visual warnings-like the ones on cigarette packs.
Not just in English. In Hindi. In Punjabi. In Tamil.
Because safety isn’t a privilege. It’s a right.
Neil Thorogood
February 2, 2026 AT 21:34So let me get this straight: we spend $7.3 billion on AI to catch what we should’ve caught in Phase 2 trials… and we still let people die because someone forgot to tell them not to eat grapefruit?
That’s not innovation. That’s just capitalism with a fancy dashboard.
Also, if your doctor didn’t ask about your supplements, fire them. 🤡
And yes, I’ve had a statin-induced rhabdo. It felt like my muscles were being shredded by a lawnmower. No thanks.
Ask the questions. Or pay the price. Your call.
Jessica Knuteson
February 2, 2026 AT 22:23Post-market surveillance is a statistical illusion. FAERS is a garbage fire of unverified reports. AI models are trained on biased data. The FDA doesn’t ‘catch’ anything. They react after the fact. And the cycle repeats.
Nothing changes.
Nothing ever will.
Just take your pills and hope.
Robin Van Emous
February 3, 2026 AT 06:37I appreciate how thorough this post is. Honestly, it’s the most balanced take I’ve read on this topic. I’m a retired nurse, and I’ve seen too many patients get hurt because no one connected the dots.
But I also think we need to be kind to each other here. Not everyone has access to a pharmacist. Not everyone speaks English. Not everyone can afford to switch meds.
Maybe the real solution isn’t just better tech-but better access. Better education. Better listening.
And maybe, just maybe, we should treat each other like people, not data points.
Thank you for writing this.
It matters.
Angie Thompson
February 3, 2026 AT 12:52Okay, real talk: I just Googled ‘atorvastatin + grapefruit’ and my heart stopped. I’ve been drinking one glass every morning for 3 years. I thought it was ‘heart-healthy.’
My doctor never said a word.
I’m canceling my next appointment and going to the pharmacy instead. I’m bringing my whole pill bottle. Every single thing.
And I’m telling everyone I know.
Because if I didn’t read this, I might’ve died quietly.
Thank you.
💖