When you pick up a prescription, you might see two options: the brand-name drug you’ve always known, or a cheaper generic version. You might wonder - are they really the same? The answer lies in two closely related but very different concepts: bioavailability and bioequivalence. Understanding the difference isn’t just for pharmacists or scientists. It’s the reason you can trust a $5 generic pill to work just like the $50 brand-name one - most of the time.
What Bioavailability Actually Means
Bioavailability tells you how much of a drug actually makes it into your bloodstream after you take it. It’s not about how strong the pill looks or how much active ingredient is listed on the label. It’s about what your body can use. For example, if you take a 100 mg tablet of a drug and only 60 mg ends up in your blood, that drug has 60% bioavailability. The rest? It’s either not absorbed from your gut, or it gets broken down by your liver before it ever reaches your bloodstream. That’s called first-pass metabolism. Bioavailability is measured using two key numbers: AUC (area under the curve) and Cmax (maximum concentration). AUC tells you how much of the drug your body is exposed to over time. Cmax tells you how high the drug spikes in your blood after you take it. These aren’t abstract terms - they’re real measurements taken from blood samples in clinical studies. There are two types of bioavailability:- Absolute bioavailability - compares how much of the drug gets into your blood after you swallow it, versus when you get it through an IV (which is 100% bioavailable because it goes straight into your veins).
- Relative bioavailability - compares two different versions of the same drug, like a brand-name tablet versus a generic tablet, both taken by mouth.
What Bioequivalence Is - and Why It Matters
Bioequivalence is the comparison game. It answers this question: “Does the generic version deliver the same amount of drug, at the same speed, as the brand-name version?” It’s not enough for a generic to have the same active ingredient. It has to behave the same way in your body. That’s where bioequivalence comes in. Regulators like the FDA, EMA, and Health Canada require that generic drugs pass a bioequivalence test before they can be sold. The test? A head-to-head study in healthy volunteers. One group takes the brand-name drug. Another group takes the generic. Blood samples are taken over 72 hours. AUC and Cmax are measured for both. Here’s the rule: the 90% confidence interval for the ratio of the generic’s AUC and Cmax compared to the brand’s must fall between 80% and 125%. This is called the 80/125 rule. Let’s say the brand drug has an average AUC of 100. The generic’s AUC must be between 80 and 125 to be considered bioequivalent. That means the generic can be up to 20% lower or 25% higher in total exposure - and still be approved. Why not 100% exactly? Because human bodies vary. Your stomach empties faster than mine. You might sweat more. Your liver enzymes work differently. The 80/125 range accounts for that natural variation - and still ensures safety.The Key Difference: One Is a Property, the Other Is a Comparison
This is where people get confused. Bioavailability is a property of a single drug product. You can measure the bioavailability of one tablet - no comparison needed. Bioequivalence is a relationship between two products. You can’t say a drug is “bioequivalent” on its own. It only makes sense when you say, “This generic is bioequivalent to the brand.” Think of it like two cars. Bioavailability tells you how much fuel each car can burn per mile. Bioequivalence tells you whether two cars use fuel at the same rate and reach the same speed under the same conditions. Both use the same measurements - AUC and Cmax. But bioequivalence adds the statistical test. It’s not just “close enough.” It’s “statistically proven to be within safe limits.”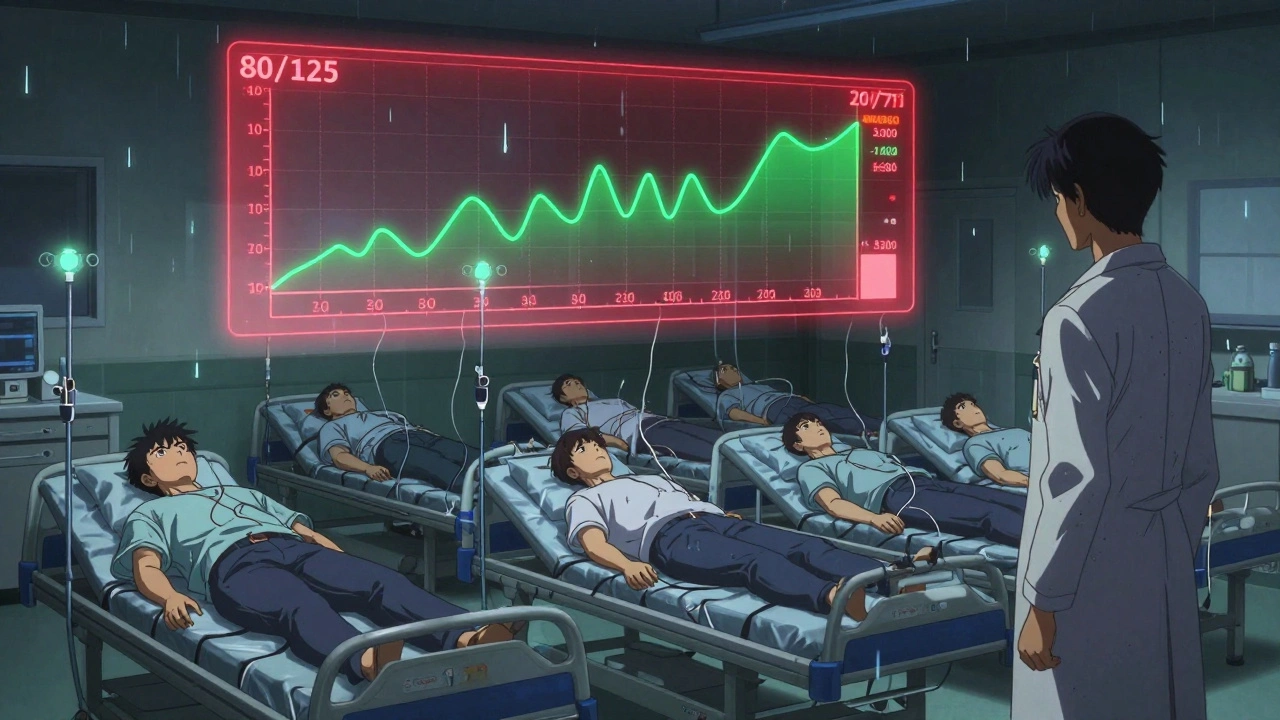
When the Rules Get Tighter
The 80/125 rule works for most drugs. But for some, even a 20% difference could be dangerous. Drugs with a narrow therapeutic index - where the difference between a helpful dose and a toxic one is tiny - need stricter rules. Think warfarin (a blood thinner), lithium (for bipolar disorder), or levothyroxine (for hypothyroidism). For these, the FDA requires tighter limits: often 90% to 111% for AUC. That’s a much smaller window. Why? Because if your thyroid hormone level drops 10% too low, you might feel exhausted. If it spikes 10% too high, you could develop heart rhythm problems. This is why some patients report issues switching generic levothyroxine brands. Even though each generic meets the 80/125 rule for the brand, switching between different generics - each with slightly different fillers or coatings - can cause small, cumulative shifts in absorption. That’s why many doctors prefer patients to stick with the same generic brand once they’ve stabilized.Real-World Data: Do Generics Actually Work?
Critics say bioequivalence studies don’t capture real-life results. But the data says otherwise. Between 2010 and 2020, 99.7% of generic drugs approved in the U.S. met bioequivalence standards. And according to the Generic Pharmaceutical Association, generics made up 91% of all prescriptions filled in 2022 - but only 22% of total drug spending. A 2023 survey by the American Pharmacists Association tracked 1,247 patients switched from brand to generic blood pressure meds. Only 17 reported issues. Of those, just 4 had true therapeutic problems - not side effects or adherence issues. That’s 0.32%. On Reddit and patient forums, people do report problems - especially with thyroid meds or seizure drugs. But when researchers look at large-scale studies, the number of confirmed bioequivalence failures is extremely low. One 2022 survey from Patients for Better Drugs found that 87.4% of respondents noticed no difference when switching to generics. Of the 12.6% who did, only 3.8% of those cases were medically confirmed as linked to bioequivalence differences. The rest? Placebo effects, stress, or other health changes.
How These Studies Are Done - And Why They’re Tricky
Bioequivalence studies aren’t simple. They’re tightly controlled. Participants are healthy adults. They fast overnight. They take one drug, then wait a week. Then they take the other. Blood is drawn every 15 to 30 minutes for up to 72 hours. That’s 12 to 18 time points per person. For a single study, you need 24 to 36 volunteers. The challenges? Food. Alcohol. Other meds. Even the time of day you take the pill can change results. Take voriconazole, an antifungal. A high-fat meal can boost its absorption by 36%. So if the brand was tested on an empty stomach, but the generic was tested with food - the results would be wrong. That’s why studies are done under fasting conditions first. Then, if the drug is usually taken with food, a second study is done with a meal. The FDA now offers a free online Bioequivalence Tool to help companies design these studies. But it still takes 3 to 6 months to run one properly. That’s why complex generics - like inhalers, creams, or injectables - are harder and more expensive to develop.What’s Next for Bioequivalence?
The field is evolving. Researchers are testing new ways to predict how a drug will behave without always needing blood tests. One promising method is physiologically-based pharmacokinetic (PBPK) modeling. It uses computer simulations based on your body’s biology - stomach pH, liver enzymes, blood flow - to predict absorption. By 2027, McKinsey predicts 30% of complex generic approvals could use this instead of full human studies. The European Union is also exploring using dissolution testing - how fast a pill breaks down in lab conditions - as a stand-in for some bioequivalence studies. If a generic dissolves exactly like the brand, maybe you don’t need 36 volunteers. But for now, blood tests are still the gold standard. And the 80/125 rule? It’s held up for over 30 years. No major safety crisis. No flood of failed generics.What You Should Know as a Patient
You don’t need to understand AUC or confidence intervals. But you should know this:- Generics are not “weaker.” They’re required to be the same - within a scientifically accepted range.
- If you’re on a narrow therapeutic index drug (like warfarin, lithium, or levothyroxine), stick with the same generic brand if possible. Don’t switch between generics unless your doctor says it’s safe.
- If you feel different after switching - fatigue, dizziness, worsening symptoms - don’t ignore it. Talk to your doctor. It’s not always the drug. But sometimes, it is.
- Cost savings are real. Generics save U.S. patients over $300 billion a year. That’s not marketing. That’s data.
Are generic drugs really as effective as brand-name drugs?
Yes, for the vast majority of drugs, generic versions are just as effective. They must meet strict bioequivalence standards set by the FDA and other global regulators, meaning they deliver the same amount of active ingredient at the same rate as the brand-name version. Studies show 99.7% of generics approved between 2010 and 2020 met these standards with no significant therapeutic differences.
What does bioequivalence mean for me as a patient?
Bioequivalence means the generic drug you’re given will work the same way in your body as the brand-name version. It’s not about the pill looking the same - it’s about how your body absorbs it. Regulators require that the generic’s absorption rate and total exposure fall within 80-125% of the brand’s. This small range accounts for natural body differences while ensuring safety and effectiveness.
Why do some people say generics don’t work as well?
Some patients report differences with drugs that have a narrow therapeutic index - like levothyroxine, warfarin, or seizure medications. Even small changes in absorption can matter. While each generic meets regulatory standards, switching between different generic brands can cause subtle shifts. That’s why doctors often recommend sticking with the same generic once you’ve stabilized. Most reported issues aren’t due to bioequivalence failure - they’re from other factors like stress, diet, or adherence.
What’s the 80/125 rule, and why does it matter?
The 80/125 rule is the standard used by regulators to determine if two drugs are bioequivalent. It means the 90% confidence interval for the ratio of the generic’s AUC and Cmax compared to the brand’s must fall between 80% and 125%. This isn’t a 20% tolerance - it’s a statistically rigorous method that accounts for how the body processes drugs multiplicatively, not additively. It ensures no more than a 20% difference in exposure, which is considered safe for most medications.
Can I switch between different generic brands?
For most drugs, yes - and you’ll likely notice no difference. But for drugs with a narrow therapeutic index - such as thyroid meds, blood thinners, or epilepsy drugs - it’s safer to stick with the same generic brand once you’ve found one that works. Switching between generics can cause small changes in absorption that add up over time. Always check with your pharmacist or doctor before switching brands, especially if you’re on a critical medication.


Martyn Stuart
December 5, 2025 AT 00:48Let me break this down simply: bioavailability is about how much of the drug actually gets into your blood; bioequivalence is about proving the generic matches the brand within a statistically valid range. The 80-125% rule isn't arbitrary-it's based on decades of pharmacokinetic data. If you're on warfarin or levothyroxine, consistency matters more than cost. Stick with one generic brand once you've stabilized. Switching between generics? That's where the real-world risks creep in-not because the FDA got it wrong, but because human biology isn't a factory line.
Jessica Baydowicz
December 5, 2025 AT 14:40OMG YES!! I switched to generic levothyroxine last year and felt like a zombie for two weeks-tired, foggy, crying at commercials. My doctor said it was ‘all in my head.’ But when I switched back to the brand? Poof. Energy returned. Turns out, even tiny absorption shifts matter when your thyroid is running the whole show. So yeah, generics are great-but not all generics are created equal. Find the one that works and DON’T let your pharmacist switch it out without asking!
Shofner Lehto
December 6, 2025 AT 16:51The 80/125 rule is a brilliant compromise. It allows for natural variation in human metabolism while ensuring therapeutic equivalence. The key is that it’s a 90% confidence interval, not a simple average. That means there’s a 90% probability the true ratio falls between 80% and 125%. This isn’t about being ‘close enough’-it’s about statistical confidence. For most drugs, this is more than sufficient. For narrow therapeutic index drugs, regulators apply tighter limits. The system works because it’s grounded in real data, not ideology.
Ben Choy
December 8, 2025 AT 02:34Just want to say thank you for writing this. I’ve been on generic blood pressure meds for five years and never thought twice about it-until my mom had a bad reaction switching generics for her heart med. Now I get it. It’s not about the pill looking different. It’s about how your body handles the fillers, coatings, and dissolution rates. I used to roll my eyes at people who said ‘the generic didn’t work.’ Now I get it. It’s not always placebo. Sometimes it’s the starch.
Emmanuel Peter
December 9, 2025 AT 03:14Okay but let’s be real-this whole bioequivalence thing is a scam. The FDA’s ‘80-125’ rule? That’s a 45% window! That means a generic could be nearly half as effective or 25% stronger and still be approved. And don’t even get me started on the fact that they test this on healthy 20-year-olds who don’t have diabetes, kidney disease, or take 12 other meds. You think your 72-year-old grandma with three chronic conditions is gonna react the same way? Please. This isn’t science-it’s corporate cost-cutting dressed up as regulation.
Ashley Elliott
December 9, 2025 AT 09:36Just a quiet note: I work in pharmacy, and I’ve seen the data. 99.7% of generics meet standards. The 0.3% that don’t? Usually because someone switched brands mid-treatment for a narrow-index drug. The system isn’t perfect, but it’s rigorously tested. If you feel off after switching, document it. Talk to your prescriber. But don’t assume it’s the drug’s fault-stress, sleep, diet, even hydration can mimic ‘medication failure.’ Most of the time, it’s not the pill. It’s the person.
Chad Handy
December 11, 2025 AT 03:39I’ve been on levothyroxine for 18 years. I’ve tried five different generics. Three of them made me feel like I was slowly drowning in molasses. One made me jittery like I’d drunk five espressos. The brand? Perfect. The last generic I tried? I could feel my heart pounding at 2 a.m. I went to my endocrinologist, and she said, ‘You’re not crazy. The generic’s dissolution profile is slightly different.’ So I pay $80 a month for the brand because my body isn’t a lab rat. The FDA says it’s ‘bioequivalent.’ But my body says, ‘Nope.’ And I’m not going back. You can’t test for how a drug makes you feel in a 72-hour study with 24 healthy volunteers. That’s not medicine-that’s statistics with a lab coat.
Augusta Barlow
December 12, 2025 AT 21:41Let’s be honest-this whole bioequivalence thing is a cover-up. The pharmaceutical companies own the FDA. They don’t want you to know that generics are often made in the same factories as the brand, but with cheaper fillers that change how the drug dissolves. And they test it on young, healthy people who don’t have the same metabolism as real patients. They don’t test for long-term effects. They don’t test with other meds. And they don’t tell you that switching between generics can cause cumulative damage over time. That’s why so many people get worse on generics. It’s not ‘placebo.’ It’s corporate greed disguised as science.
Chase Brittingham
December 13, 2025 AT 19:19Shofner nailed it. The 80/125 rule isn’t a loophole-it’s a safety buffer. Human bodies aren’t robots. Your liver enzymes vary. Your gut pH changes. You eat differently than the guy in the study. The rule accounts for that. And if you’re on warfarin? Don’t switch generics unless your INR is monitored. That’s not fear-mongering-that’s clinical practice. The system works. It’s not perfect, but it’s the best we’ve got. And it saves billions. That’s worth supporting.
Bill Wolfe
December 15, 2025 AT 11:30Wow. Just… wow. You people are so naive. You think the FDA cares about your thyroid? They care about profits. The 80/125 rule was designed by Big Pharma lobbyists. They knew they could get away with a 25% higher absorption window because most people won’t notice-until they develop atrial fibrillation from a 15% spike in levothyroxine. And don’t get me started on the fact that 80% of generics are made in India and China, where inspections are a joke. This isn’t science. It’s a Ponzi scheme disguised as healthcare.
Ollie Newland
December 16, 2025 AT 01:36For the record: bioequivalence is a PK/PD model, not a regulatory checkbox. AUC and Cmax are pharmacokinetic endpoints, but they’re surrogates for pharmacodynamic outcomes-i.e., clinical effect. The 80-125% CI is derived from log-transformed data because drug exposure follows a multiplicative, not additive, model. That’s why a 20% difference in AUC isn’t the same as a 20% difference in dose. It’s math. It’s biology. And for most drugs? It’s sufficient. For NTDs? Tighter limits. The system’s elegant. It’s not perfect, but it’s the most rigorously validated framework we have. Stop blaming regulators. Start understanding the science.